LDAA Metabolite Calculator
This tool estimates the metabolic shifts when combining azathioprine with allopurinol (LDAA protocol). It calculates:
- Appropriate low-dose azathioprine (50mg)
- Expected 6-MMP reduction (70-90%)
- Expected 6-TGN increase (2-5x)
IMPORTANT: This is for educational purposes only. Always follow your doctor's guidance and therapeutic drug monitoring.
Your Estimated Metabolite Levels
pmol/8×10⁸ RBCs
pmol/8×10⁸ RBCs
For optimal results:
- 6-MMP: Below 2,800
- 6-TGN: 230-450
This combination is only safe under medical supervision:
- Weekly blood tests for first 4 weeks
- Contraindicated with severe kidney impairment (creatinine clearance < 30 mL/min)
- TPMT activity must be checked before starting
When azathioprine doesn’t work-or makes you sicker-doctors have a secret weapon: combining it with allopurinol. This isn’t a random mix. It’s a precise, science-backed fix for a hidden problem: toxic metabolite accumulation. For patients with inflammatory bowel disease (IBD) or autoimmune hepatitis who can’t tolerate standard azathioprine doses, this combo can mean the difference between remission and hospitalization.
Why Azathioprine Alone Can Be Dangerous
Azathioprine has been used since the 1960s to calm overactive immune systems. It’s cheap, effective, and works for many. But not everyone responds the same way. About 15-20% of patients are "hypermethylators"-their bodies convert too much azathioprine into 6-methylmercaptopurine (6-MMP). That’s not the good kind of metabolite. 6-MMP builds up in the liver, causing elevated enzymes, nausea, and sometimes full-blown hepatitis. Meanwhile, the therapeutic metabolite, 6-thioguanine nucleotides (6-TGN), stays too low to fight inflammation. Patients feel worse, not better. And their doctors are stuck: raise the dose, and risk liver damage. Lower it, and the disease flares.How Allopurinol Changes the Game
Allopurinol was invented to treat gout. It blocks an enzyme called xanthine oxidase, which breaks down purines. But here’s the twist: xanthine oxidase also breaks down azathioprine’s active form, 6-mercaptopurine (6-MP). When you block that enzyme with allopurinol, the body can’t make as much 6-MMP. Instead, it’s forced down a different path-toward 6-TGN. That’s the metabolite that actually suppresses the immune system and helps heal the gut. This shift is dramatic. Studies show 6-MMP levels drop by 70-90%. 6-TGN levels rise 2 to 5 times. For hypermethylators, this isn’t just helpful-it’s life-changing. One patient on Reddit described how his liver enzymes, which had been sky-high for three years, returned to normal within eight weeks of starting the combo. He’s been in remission for over a year.The LDAA Protocol: Less Azathioprine, More Safety
You can’t just take both drugs at full dose. That’s how people end up in the hospital. The key is low-dose azathioprine with allopurinol-called LDAA. The standard is 50 mg of azathioprine per day (instead of 150-200 mg) and 100 mg of allopurinol daily. This reduces the risk of bone marrow suppression while still delivering enough 6-TGN to work. But timing matters. Blood counts must be checked weekly for the first four weeks. Why? Because even with the right dose, some people develop low white blood cells-neutropenia-around weeks 4 to 8. It’s not common, but it happens. And if you miss it, you risk serious infection. One patient posted on Reddit about dropping his absolute neutrophil count to 0.8. He was hospitalized with fever. He didn’t get his blood tested. That’s the danger.
Who Benefits Most?
Not everyone is a candidate. LDAA works best for people with high thiopurine methyltransferase (TPMT) activity-those who naturally make too much 6-MMP. About 30-40% of IBD patients fall into this group. If you’ve tried standard azathioprine and had liver problems, you’re likely one of them. But if you have low TPMT activity (less than 5 U/mL), LDAA won’t help. You’re already at high risk for bone marrow suppression. Giving you allopurinol won’t fix that-it could make it worse. These patients need other options, like biologics. Therapeutic drug monitoring is non-negotiable. After four weeks, your doctor should check your red blood cell levels for 6-TGN and 6-MMP. The sweet spot? 6-TGN between 230 and 450 pmol per 8×10⁸ red blood cells. Too low? The drug isn’t working. Too high? You’re in danger of myelosuppression. 6-MMP should be under 2,800 pmol. If it’s not, the combo isn’t working right.Real Results, Real Risks
The data speaks for itself. In a 2019 meta-analysis, patients on LDAA had remission rates of 65-75%. Standard azathioprine? Only 30-40%. In a 2018 study of 42 patients with liver toxicity, 85-90% saw their enzymes return to normal. That’s not luck. That’s metabolism being redirected. But the risks are real. Before proper protocols were established, early studies saw leukopenia rates as high as 40%. Today, with strict monitoring, that’s down to 5-10%. The difference? Awareness. Testing. Dose control. The American Gastroenterological Association and the European Crohn’s and Colitis Organisation now recommend LDAA for patients who can’t tolerate standard azathioprine. It’s not experimental. It’s guideline-backed. Yet, adoption varies. In Europe, 65% of IBD centers use it. In the U.S., only 35% of community practices do. Why? Fear. Outdated warnings. Lack of training.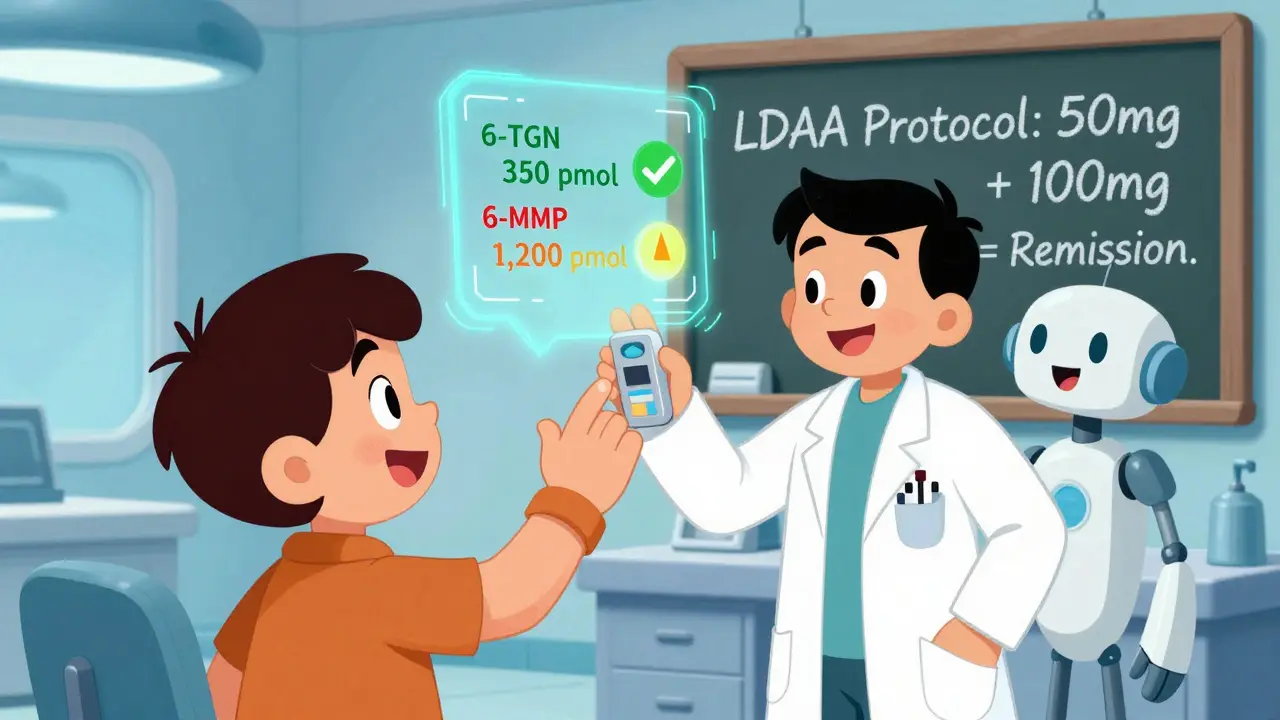
Cost and Accessibility
Azathioprine costs about $20 a month. Allopurinol? Less than $10. Together, it’s under $1,800 a year. Compare that to biologics like Humira or Remicade-$30,000 to $50,000 annually. For patients without good insurance, or in countries with limited healthcare access, LDAA isn’t just smart-it’s essential. It’s also being tested beyond IBD. A 2023 study showed 82% of autoimmune hepatitis patients responded to LDAA after failing standard therapy. That’s promising. More research is coming.What You Need to Know Before Starting
If your doctor suggests LDAA, ask these questions:- Have I been tested for TPMT activity?
- What were my 6-MMP and 6-TGN levels before starting?
- Will I get weekly blood tests for the first month?
- When will you check my metabolite levels again?
- What happens if my white blood cell count drops?
What Comes Next?
The future of LDAA is faster testing. Two companies are developing point-of-care devices that can measure 6-TGN and 6-MMP in under an hour. Right now, results take days. Imagine walking into your GI clinic, getting a finger prick, and knowing your dose is right before you leave. That’s coming soon. For now, LDAA remains one of the most underused, high-impact tools in gastroenterology. It doesn’t require fancy infusions or expensive patents. Just knowledge. Careful dosing. And relentless monitoring.If you’ve been stuck on azathioprine with no progress-or worse, side effects-ask your doctor about LDAA. It might be the missing piece.
Can I take azathioprine and allopurinol together without a doctor’s supervision?
No. Combining azathioprine and allopurinol without medical supervision is dangerous. Allopurinol changes how your body processes azathioprine, which can lead to severe bone marrow suppression. This combination requires strict dosing (azathioprine at 25-33% of the usual dose), weekly blood tests for at least four weeks, and therapeutic drug monitoring. Never adjust these medications on your own.
What are the signs that LDAA isn’t working?
If your symptoms like diarrhea, abdominal pain, or fatigue haven’t improved after 8-12 weeks, or if your liver enzymes remain elevated, LDAA may not be working. The most reliable way to know is through therapeutic drug monitoring. If your 6-TGN levels are below 230 pmol/8×10⁸ RBCs, the dose is too low. If your 6-MMP is still above 2,800 pmol/8×10⁸ RBCs, the metabolic shift isn’t happening as expected. Talk to your doctor about adjusting the dose or switching strategies.
Is LDAA safe for patients with kidney problems?
No. LDAA is contraindicated in patients with severe kidney impairment (creatinine clearance under 30 mL/min). Allopurinol and its active metabolite, oxypurinol, are cleared by the kidneys. If your kidneys aren’t working well, these drugs build up in your system, increasing the risk of toxicity-even at standard doses. Your doctor should check your kidney function before starting LDAA and avoid it if your creatinine clearance is too low.
How long does it take to see results with LDAA?
Improvement varies. Liver enzyme levels often normalize within 4 to 8 weeks. Clinical symptoms like reduced bowel movements or less abdominal pain may take 8 to 12 weeks. Full remission can take up to 6 months. The key is patience and monitoring. Don’t stop the medication just because you don’t feel better right away-unless you have signs of infection or severe fatigue, which could mean low blood counts.
Can I switch back to regular azathioprine after LDAA?
It’s not recommended. Once you’ve switched to LDAA and your body has adapted to the new metabolic pathway, going back to full-dose azathioprine can trigger a dangerous spike in 6-MMP and liver toxicity. Most patients stay on the combination long-term. If you need to stop, your doctor will guide you through a safe transition to another therapy, like a biologic or methotrexate-not back to standard azathioprine.
Does LDAA increase cancer risk?
Azathioprine alone carries a small increased risk of lymphoma and skin cancer due to long-term immunosuppression. LDAA doesn’t add to that risk-it may even reduce it by allowing lower overall drug exposure. However, the risk isn’t zero. Patients on LDAA should still practice sun safety, get regular skin checks, and report unusual lumps or persistent infections. The benefit of controlling active disease usually outweighs this small risk, especially when compared to uncontrolled inflammation, which itself increases cancer risk.

Keith Oliver
So you're telling me we're just repurposing a gout drug to fix a metabolite problem we created with a 60-year-old immunosuppressant? Classic pharma spaghetti theory. I've seen this before - they throw two old drugs together, call it innovation, and charge nothing while biologics make billions. Someone's got a spreadsheet somewhere.