When you order medication online, you’re trusting a website with your health. But not all online pharmacies are created equal. Some are legitimate, licensed, and safe. Others sell fake pills, dangerous dosages, or no medicine at all. In 2025, the FDA and state pharmacy boards are tightening their grip on this growing industry-especially as telemedicine and compounded drugs like Semaglutide flood the market. Understanding how federal and state regulators work together-or sometimes step on each other’s toes-isn’t just for pharmacists or lawyers. It’s for anyone who’s ever clicked "Buy Now" on a prescription drug.
What the FDA Actually Controls
The FDA doesn’t license online pharmacies. That’s not its job. Instead, it watches the drugs themselves. If a website sells a medication that hasn’t been approved by the FDA, it’s breaking the law-even if the pharmacy has a U.S. address and a licensed pharmacist on staff. The agency focuses on three big red flags: unapproved drugs, no prescription required, and misleading advertising.
In the first nine months of 2025, the FDA issued 147 warning letters to illegal online pharmacies. That’s a 32% jump from 2024. Many of these sites were selling counterfeit versions of popular weight-loss drugs like Tirzepatide or diabetes medications. Some pills had too much active ingredient. Others had none. A few even contained rat poison or industrial chemicals. The FDA’s BeSafeRx tool, which lets you check if a pharmacy is licensed by a state board, saw over 1.2 million visits in Q3 2025 alone. It’s not perfect-but it’s the best public defense most people have.
Another major focus is advertising. In September 2025, the FDA and HHS announced new rules targeting direct-to-consumer ads on social media. Influencers promoting GLP-1 drugs without mentioning side effects like nausea, pancreatitis, or thyroid cancer are now on the radar. The FDA’s Office of Prescription Drug Promotion, which used to issue just five warning letters a year in 2024, is now ramping up enforcement. They’re scanning Instagram posts, YouTube videos, and TikTok ads for missing risk disclosures.
State Boards: The Real Gatekeepers
While the FDA checks the drugs, state boards of pharmacy check the people. Every legitimate online pharmacy must be licensed by the state where it’s physically located. There are 50 states, each with its own rules. Some require pharmacists to verify a patient’s identity with a government ID before dispensing a controlled substance. Others mandate that telemedicine prescriptions be reviewed by a pharmacist within 24 hours. A few even ban certain types of compounding.
As of November 2025, 48 out of 50 states offer public online databases where you can search for licensed pharmacies. California, Texas, and Florida had the most complaints about online pharmacies in 2024-mostly because they have the biggest populations and the most aggressive enforcement teams. If a pharmacy operates in Florida but ships to Ohio, it still needs a Florida license. And if it doesn’t, the Ohio board can’t touch it. That’s where federal agencies step in.
The National Association of Boards of Pharmacy (NABP) runs the VIPPS program, which accredits online pharmacies that meet strict standards. As of October 2025, only 187 pharmacies held VIPPS accreditation. That’s less than 1% of the estimated 20,000 online pharmacies operating in the U.S. But if you see the VIPPS seal, you know the pharmacy has passed background checks, uses secure payment systems, and has a real, verifiable physical address.
The DEA’s New Telemedicine Rules
The DEA controls controlled substances-drugs like opioids, stimulants, and sedatives. Before 2025, the Ryan Haight Act required an in-person visit before prescribing these drugs online. But the pandemic changed everything. Temporary rules allowed telehealth prescriptions without a physical exam. Now, those rules are being replaced with something more structured.
In January 2025, the DEA announced three new Special Registrations for telemedicine providers:
- Standard Registration: For Schedule III-V drugs (like hydrocodone or Xanax). Providers must check the patient’s state PDMP data before prescribing.
- Advanced Registration: For Schedule II drugs (like oxycodone or Adderall). Only psychiatrists, hospice doctors, pediatricians, and long-term care physicians qualify-and they need board certification.
- Limited State Registration: For providers who only prescribe in one state, following that state’s specific rules.
This is a major shift. It’s no longer about whether you’ve met the patient in person. It’s about whether you’re registered, trained, and checking the right databases. The DEA is also building a nationwide Prescription Drug Monitoring Program (PDMP) to replace the current patchwork of 50 separate state systems. By Q3 2026, pharmacists and doctors should be able to see a patient’s full prescription history across state lines.

Compounded Drugs: The Gray Zone
When Semaglutide and Tirzepatide went into shortage in 2024, demand exploded. Compounding pharmacies-those that mix custom doses-stepped in. But here’s the catch: compounded drugs are not FDA-approved. That means the FDA doesn’t test them for safety, strength, or purity before they’re sold.
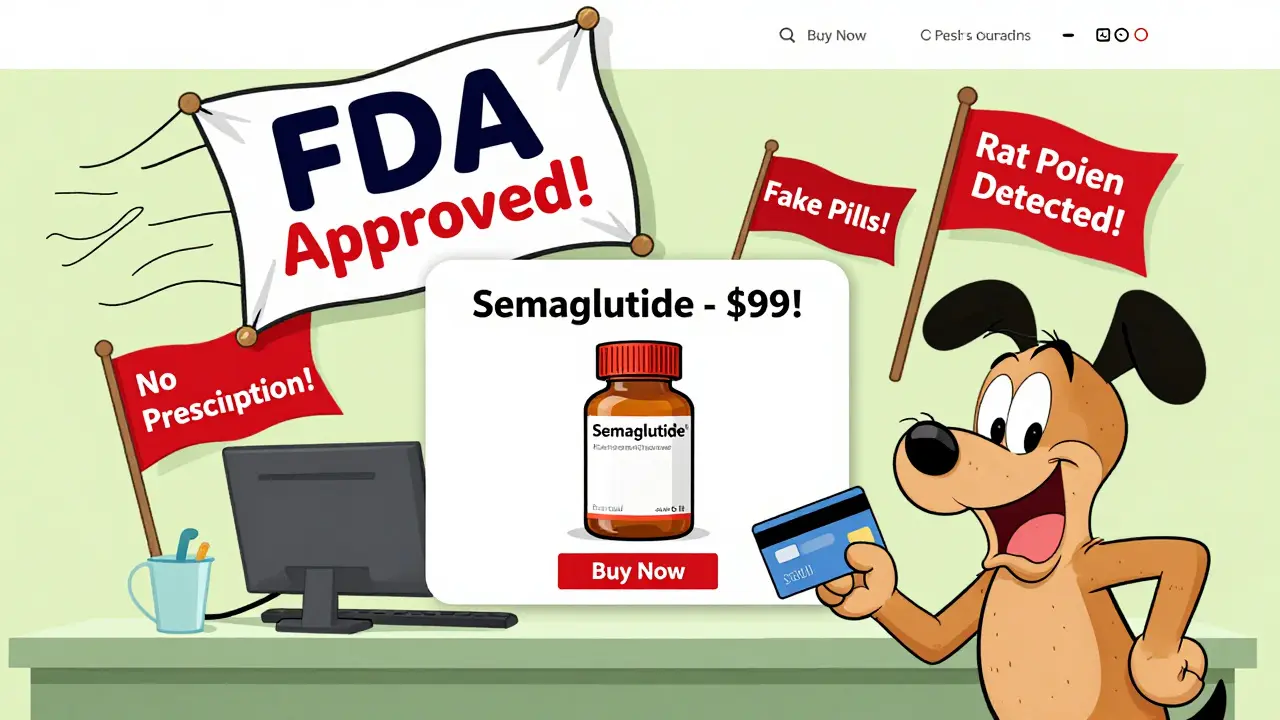
Only 503A compounding pharmacies are allowed to fill patient-specific prescriptions. 503B facilities, which make bulk batches for clinics, are more tightly regulated-but they can’t legally sell directly to consumers. In September 2025, the FDA clarified that 503A pharmacies must still follow state laws, get valid prescriptions, and avoid mass-producing drugs that are commercially available. Yet many online pharmacies ignored these limits. In August 2025, the FDA fined QuickMedsOnline.com $500,000 for selling compounded GLP-1 drugs without proper prescriptions.
Patients don’t always know the difference. They see a website selling "FDA-approved Semaglutide" for $99 a month and assume it’s safe. It’s not. The FDA doesn’t approve compounded versions. The state board might license the pharmacy, but that doesn’t mean the drug is safe.
What You Should Do
If you’re buying medication online, here’s your checklist:
- Check the BeSafeRx tool. Go to the FDA’s website and enter the pharmacy’s name or address. If it doesn’t show up, walk away.
- Look for the VIPPS seal. Click it. It should link to the NABP’s verification page.
- Verify the prescription requirement. No legitimate pharmacy will sell you a controlled substance without a valid prescription from a licensed provider.
- Check the state board database. Even if BeSafeRx says it’s okay, go to your state’s pharmacy board site and search for the pharmacy by name.
- Watch for red flags. Prices that seem too good to be true? No phone number? No physical address? No licensed pharmacist available to answer questions? That’s not a pharmacy. That’s a scam.
Most people who use online pharmacies safely are using services from big names like CVS, Walgreens, or Kaiser Permanente. In 2025, 78% of online pharmacy users chose platforms tied to traditional brick-and-mortar stores. These companies have compliance teams, auditors, and legal departments that make sure they follow every rule. They’re not perfect-but they’re far safer than random websites.

Why This Matters
Regulatory oversight isn’t about stopping innovation. It’s about stopping deaths. In 2024, the CDC reported 20 cases of patients hospitalized after taking counterfeit GLP-1 drugs sold online. One woman in Ohio ended up in the ICU after taking a pill that contained 10 times the intended dose of semaglutide. Another man in Texas lost vision after using a compounded tirzepatide that had unknown contaminants.
At the same time, patients in rural areas rely on telemedicine to get medications they couldn’t otherwise access. The DEA’s new rules try to balance that. A patient in Montana with chronic pain shouldn’t have to drive three hours to see a doctor. But they shouldn’t get a prescription from a bot on a website that doesn’t check their history either.
The system is messy. Federal agencies set broad rules. States enforce them differently. Compounding pharmacies operate in legal gray areas. And the internet makes it easy for bad actors to hide.
But you don’t need to understand all the complexity to stay safe. Just remember: if it looks too easy, too cheap, or too fast-it probably is. And if you’re unsure, call your local pharmacist. They’ll tell you whether a website is legit. They’ve seen the bad ones. They know what to look for.
How can I tell if an online pharmacy is legitimate?
Use the FDA’s BeSafeRx tool to check if the pharmacy is licensed by a state board. Look for the VIPPS seal from the National Association of Boards of Pharmacy. A legitimate pharmacy will always require a prescription, provide a U.S. physical address and phone number, and have a licensed pharmacist available to answer questions. If any of these are missing, it’s not safe.
Can I buy controlled substances like Xanax or Adderall online?
You can only buy them legally if the pharmacy is DEA-registered and you have a valid prescription from a provider who’s also DEA-registered under the new telemedicine rules. The provider must check your state’s Prescription Drug Monitoring Program (PDMP) before prescribing. Any site that sells these drugs without a prescription or without requiring a telehealth consultation is breaking the law.
Are compounded drugs like Semaglutide safe to buy online?
Compounded drugs are not FDA-approved, so their safety isn’t guaranteed. Only 503A compounding pharmacies can legally fill patient-specific prescriptions. Even then, they must follow state rules and use verified prescriptions. Avoid websites that sell compounded drugs in bulk, claim they’re "FDA-approved," or offer them at prices far below retail. Many contain incorrect dosages or harmful ingredients.
Why do some online pharmacies have U.S. addresses but still sell illegal drugs?
Some illegal pharmacies use fake U.S. addresses or rent mailboxes to appear local. The FDA and DEA can’t shut them down unless they can prove the pharmacy is operating illegally-like selling without prescriptions or distributing unapproved drugs. Many operate from overseas servers or use shell companies. That’s why checking the pharmacy’s license through your state board is more reliable than trusting the address on the website.
What should I do if I received a dangerous or fake drug from an online pharmacy?
Stop taking the medication immediately. Contact your doctor or go to the ER if you have side effects. Report the pharmacy to the FDA through their MedWatch program and to your state board of pharmacy. You can also file a complaint with the DEA if the drug was a controlled substance. Keep the packaging, receipts, and any communication with the pharmacy-they’ll help with investigations.
What’s Next for Online Pharmacy Regulation
By the end of 2026, the DEA plans to fully launch its nationwide PDMP system. That will let pharmacists and doctors see a patient’s full prescription history-no matter which state they’re in. The FDA also plans to add real-time verification to BeSafeRx, so you’ll be able to confirm if a telemedicine prescription is valid before the pharmacy fills it.
Expect more enforcement against social media ads. The FDA is already tracking influencer posts and shutting down accounts that promote drugs without proper warnings. Compounded drug oversight will also tighten, especially around GLP-1 medications. Analysts predict a 22% increase in FDA warning letters in 2026, mostly targeting websites that mislead consumers about safety or efficacy.
For now, the safest bet is simple: stick with pharmacies you know. CVS, Walgreens, and other major chains have invested millions in compliance. They’re not perfect, but they’re accountable. If you need to go elsewhere, verify. Always. Because when it comes to your health, a $50 discount isn’t worth a trip to the hospital.

Adam Rivera
Just last week I ordered my metformin from CVS’s online portal-took three days, cost $12, and I got a call from a real pharmacist asking if I was having any side effects. Wild, right? Meanwhile, my cousin bought ‘Semaglutide’ off some TikTok ad for $40 and ended up in the ER with liver enzymes through the roof. Stick with the big names. Seriously.