When you pick up a prescription and see a generic version on the shelf, you might wonder: Is this really the same thing? It’s not just about saving money-though that matters a lot. It’s about whether the pill in your hand will work the same way as the brand-name drug your doctor originally prescribed. The answer lies in something called pharmaceutical equivalence. And understanding what it actually means can help you make smarter choices about your meds.
What Pharmaceutical Equivalence Actually Means
Pharmaceutical equivalence is a technical term, but it boils down to one simple idea: same active ingredient, same dose, same form. If a generic drug is pharmaceutically equivalent to the brand-name version, it contains the exact same active pharmaceutical ingredient (API) in the same strength and dosage form-like a 10mg tablet, a 500mg capsule, or a 10mL injectable solution.
The U.S. Food and Drug Administration (FDA) sets this standard. It’s the first gate a generic drug has to pass before anyone even checks if it works the same way in your body. The FDA requires that the generic matches the brand-name drug in:
- Active ingredient (type and amount)
- Dosage form (tablet, liquid, patch, etc.)
- Route of administration (oral, injected, inhaled)
- Strength (e.g., 20mg, 500mg)
- Purity, quality, and identity of the active ingredient
That’s it. No more, no less. That’s what pharmaceutical equivalence guarantees. It doesn’t say anything about how fast the drug gets into your bloodstream. It doesn’t say anything about how you feel after taking it. It just says: this pill has the same medicine in it, the same amount, in the same shape.
What’s Not Required to Be the Same
Here’s where people get confused. Just because two pills are pharmaceutically equivalent doesn’t mean they look or taste the same.
Generics can-and often do-differ in:
- Color, shape, or size
- Flavoring or coating
- Fillers, binders, and preservatives (called excipients)
- Packaging
These differences are allowed because they don’t affect the active ingredient. But they can matter. For example, some people are allergic to certain dyes or lactose, which might be in one brand but not another. A 2022 survey found that 87% of pharmacists have had at least one patient react to an excipient in a generic drug. Most reactions are mild-stomach upset, rash-but for some, switching generics can mean switching back to the brand name.
So if your generic looks different from last month’s, that’s normal. But if you notice new side effects after switching, talk to your pharmacist. It might not be the active ingredient-it could be the filler.
Pharmaceutical Equivalence Isn’t Enough
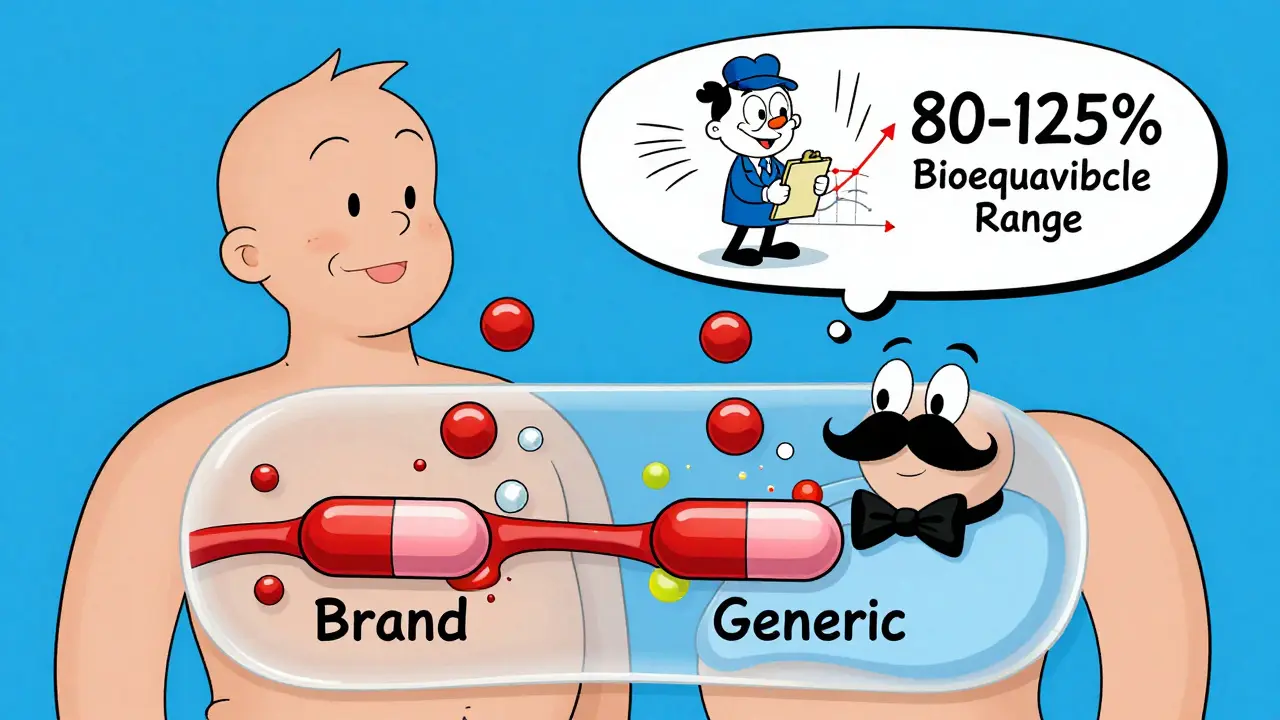
Here’s the critical point: pharmaceutical equivalence is just the starting line. It doesn’t mean the drug will work the same in your body. That’s where bioequivalence comes in.
Think of it like two identical cars with the same engine. One might have a better fuel pump or a different air filter. They’re the same on paper, but one might accelerate faster or run hotter. That’s the difference between pharmaceutical and bioequivalence.
Bioequivalence means the generic drug gets into your bloodstream at the same rate and in the same amount as the brand-name drug. The FDA requires this to be within 80% to 125% of the brand’s levels. That’s a wide range, but it’s based on real human variability. Your body absorbs drugs differently based on what you ate, your liver function, even your gut bacteria.
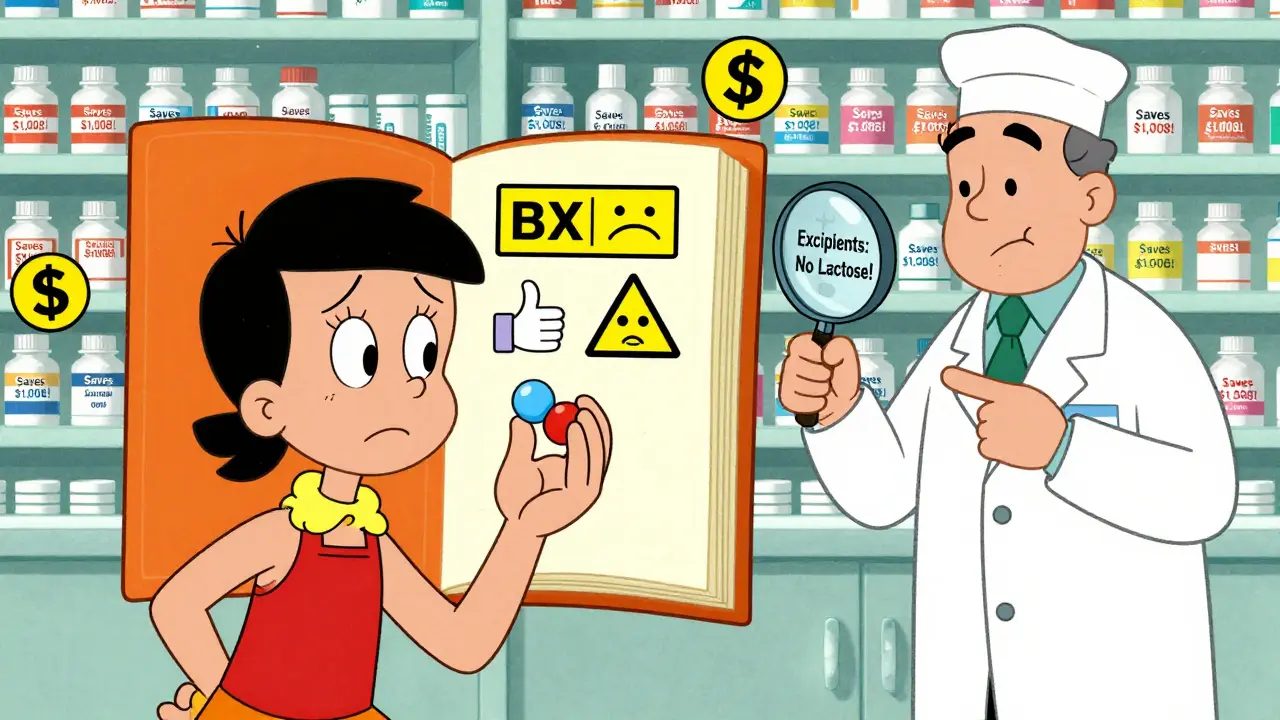
For most drugs, this range is perfectly safe. But for drugs with a narrow therapeutic index-like warfarin, lithium, or levothyroxine-tiny differences in absorption can cause big problems. That’s why pharmacists and doctors are extra careful when substituting these. The FDA’s Orange Book rates these drugs as “AB” if they’re therapeutically equivalent, and “BX” if they’re not. As of June 2024, over 12,800 generic drugs were rated “AB,” meaning they’re considered safe to swap. But over 2,500 were not.
Why This Matters in Real Life
You’re not just buying a pill. You’re buying safety, consistency, and trust.
When a pharmacy fills your prescription with a generic, they rely on the Orange Book. That’s the FDA’s official list of approved generics and their therapeutic ratings. Most pharmacies automatically substitute generics unless your doctor writes “dispense as written.” And for good reason: in 2023, 90% of all prescriptions in the U.S. were filled with generics.
The savings are huge. From 2009 to 2023, generic drugs saved the U.S. healthcare system over $2.2 trillion. On average, you save $1,008 per prescription when you take a generic instead of the brand. That’s not just a discount-it’s access to medicine.
But here’s the catch: many patients still don’t understand the difference between pharmaceutical and bioequivalence. A 2023 Kaiser Family Foundation survey found that 42% of people think generics contain only 80% of the active ingredient. That’s not true. The 80-125% range refers to how much of the drug ends up in your blood-not how much is in the pill.
That misunderstanding causes fear. And fear leads to people refusing generics-even when they’re safe and effective.
What You Should Do When Switching to a Generic
Switching to a generic isn’t a gamble. It’s a science-backed decision. But you can make it even safer.
Here’s what to do:
- Ask your pharmacist if the generic is rated “AB” in the Orange Book. If it is, it’s considered therapeutically equivalent.
- Check the pill. If it looks different from your last prescription, that’s normal. But if you notice new side effects, don’t ignore them.
- Keep a log. Note when you switch, what you’re taking, and any changes in how you feel. This helps your doctor spot patterns.
- Speak up if you’re on a narrow therapeutic index drug. If you’re on warfarin, thyroid meds, or seizure drugs, ask your doctor if the generic you’re getting is known to be interchangeable.
- Know your excipients. If you have allergies to dyes, gluten, or lactose, ask your pharmacist for the full ingredient list. Most pharmacies can provide this on request.
The Future of Generic Drugs
The system isn’t perfect. For complex drugs-like inhalers, topical creams, or injectables-pharmaceutical equivalence alone isn’t enough to guarantee the same result. That’s why the FDA launched its Complex Generic Drug Product Development program in 2023. They’re now requiring more advanced testing, like Raman spectroscopy and X-ray diffraction, to check the physical structure of the drug beyond just the chemical content.
Also, in May 2024, the FDA proposed new rules that would require generic makers to disclose more about excipients that affect how the drug releases in the body. That’s a big step toward transparency.
Right now, 97% of U.S. pharmacies automatically substitute generics. But as more complex drugs go generic, the rules will keep evolving. The goal? To make sure every generic, no matter how complicated, works just as well as the brand.
Bottom Line
Pharmaceutical equivalence means your generic has the same medicine in the same dose as the brand. That’s the foundation. But it’s not the whole story. Bioequivalence and therapeutic equivalence are what ensure it works the same way in your body.
For most people, generics are safe, effective, and a smart financial choice. But if you’re on a critical medication, have allergies, or notice changes after switching, don’t assume it’s all the same. Ask questions. Check the Orange Book. Talk to your pharmacist. You’re not just saving money-you’re taking control of your health.


Brenda King
So many people think generics are just cheap knockoffs, but this breakdown is spot on. Same active ingredient, same dose, same form-that’s the law. I’ve been on levothyroxine for 12 years and switched generics three times. No issues, as long as it’s AB-rated. Just don’t switch brands randomly if you’re sensitive. 🙏