Gut Health Risk Assessment Tool
Assess Your Gut Health Risk
This tool helps you understand your potential risk of malabsorption-related autoimmune issues based on your symptoms, diet, and lifestyle factors. Your results will provide personalized insights to support your health journey.
Important Note: This is not a diagnostic tool. For medical advice, please consult a healthcare professional.
Your Gut Health Risk Assessment
Low Risk
Key Risk Indicators
Personalized Recommendations
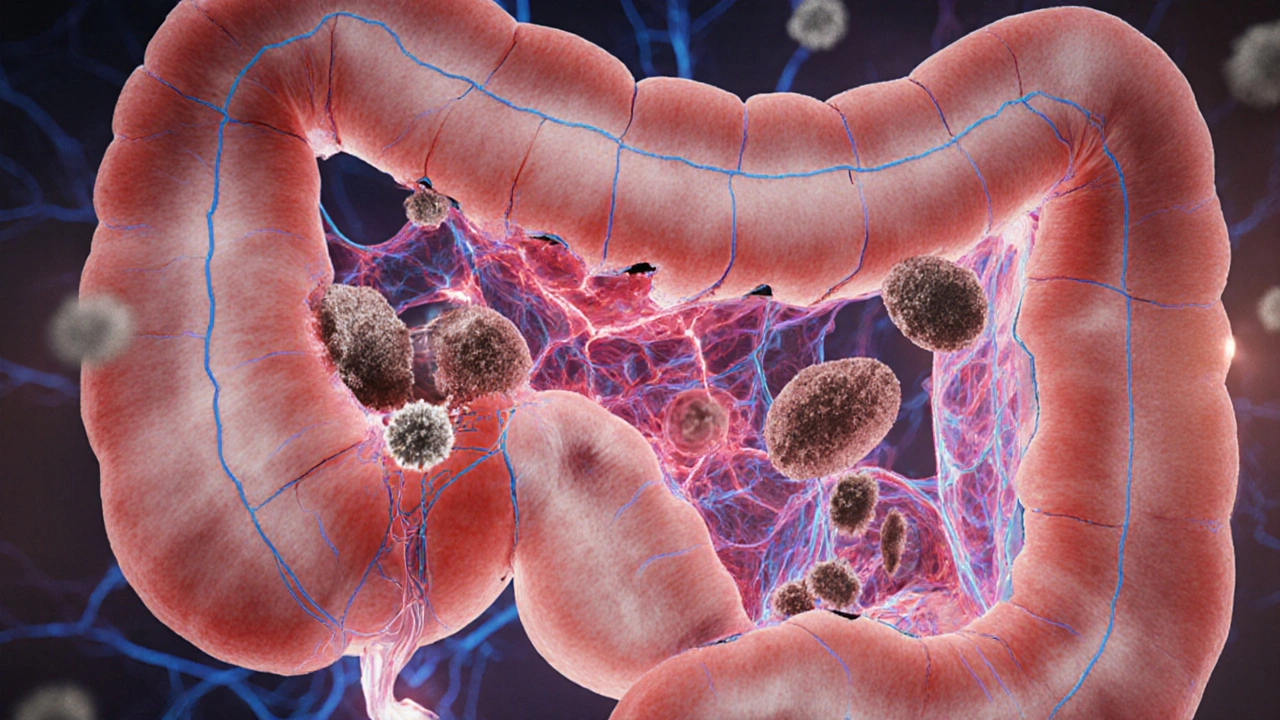
When malabsorption is the condition where the digestive system fails to break down or absorb essential nutrients from food, it does more than cause fatigue or weight loss - it can set the stage for the immune system to turn against the body.
Quick Takeaways
- Malabsorption disrupts gut barrier integrity, often leading to "leaky gut".
- A compromised barrier lets undigested food particles and microbes access the bloodstream, triggering immune responses.
- Common culprits include celiac disease, inflammatory bowel disease (IBD), and chronic dysbiosis.
- Addressing nutrient deficiencies, restoring microbiome balance, and reducing gut inflammation can lower autoimmune risk.
How Food Absorption Breaks Down
Digestive absorption hinges on three steps: breakdown, transport, and uptake. Enzymes shred proteins, fats, and carbs; the gut lining’s villi and microvilli act as highways, and transport proteins shuttle nutrients into blood vessels. Any disruption-whether from enzyme deficiencies, damaged villi, or altered transporter expression-creates a bottleneck.
Factors that impair this process include:
- Enzyme shortfalls (e.g., low lactase, pancreatic insufficiency).
- Inflammation that flattens villi, as seen in celiac disease.
- Microbial imbalances that produce toxins interfering with transporter function.
Why the Immune System Gets Involved
The gut houses roughly 70% of the body’s immune cells. When the barrier weakens, the immune system perceives escaped food antigens and bacterial fragments as threats. This misidentification can spark chronic inflammation and, over time, the development of autoimmune disorder a condition where the body’s immune response mistakenly attacks its own tissues.
Key mechanisms include:
- Molecular mimicry: Some food proteins resemble body proteins, confusing immune cells.
- Activation of Th17 cells, which drive auto‑inflammatory pathways.
- Release of cytokines (IL‑6, TNF‑α) that sustain systemic inflammation.

Gut Microbiome: The Missing Link
The collection of trillions of microbes-collectively known as the gut microbiome refers to the community of bacteria, fungi, and viruses living in the digestive tract-plays a decisive role in nutrient extraction and immune education. When diversity drops (a state called dysbiosis), the microbiome loses its ability to produce short‑chain fatty acids that reinforce the gut lining.
Research from 2023 showed that patients with newly diagnosed rheumatoid arthritis had a 30% reduction in beneficial Bifidobacterium species compared to healthy controls, highlighting the microbiome’s contribution to autoimmunity.
Leaky Gut Syndrome: When the Barrier Fails
Often described as "increased intestinal permeability", leaky gut occurs when tight junction proteins (e.g., zonulin) become loose. This permits large molecules-undigested gluten fragments, bacterial lipopolysaccharides-to slip into circulation.
Once there, they act as adjuvants, amplifying immune activation. Studies link elevated zonulin levels with both type 1 diabetes and multiple sclerosis, underscoring the clinical relevance.
Common Conditions Linking Malabsorption and Autoimmunity
Three disorders epitomize the malabsorption‑autoimmunity connection:
- Celiac disease an autoimmune reaction to gluten that damages the small‑intestine villi
- Inflammatory bowel disease (IBD) includes Crohn's disease and ulcerative colitis, both causing chronic gut inflammation and nutrient malabsorption
- Leaky gut syndrome a functional condition where intestinal permeability is increased, often secondary to other diseases
| Aspect | Celiac Disease | IBD (Crohn’s/UC) |
|---|---|---|
| Primary Trigger | Gluten (wheat, barley, rye) | Genetic & environmental (microbiome, smoking) |
| Typical Malabsorption | Iron, calcium, folate | Fat‑soluble vitamins, B12 |
| Associated Autoimmune Conditions | Type 1 diabetes, thyroiditis | Primary sclerosing cholangitis, ankylosing spondylitis |
| Key Biomarker | Anti‑tTG IgA | CRP, fecal calprotectin |

Other Influencing Factors
Beyond the main diseases, several lifestyle and genetic elements sway the malabsorption‑autoimmunity axis.
- Vitamin D deficiency low circulating 25‑hydroxyvitamin D, which modulates immune tolerance is linked to higher rates of multiple sclerosis and lupus.
- HLA‑DQ2/DQ8 genes increase susceptibility to celiac disease, shaping how the immune system reacts to gluten.
- Chronic stress raises cortisol, which can thin the gut lining and alter microbiome composition.
Testing and Managing the Connection
Identifying the root cause requires a combination of lab work and functional assessments.
- Serology: anti‑tTG IgA for celiac, anti‑endomysial antibodies, and tissue transglutaminase tests.
- Stool analysis: evaluates dysbiosis, short‑chain fatty acid output, and calprotectin levels.
- Intestinal permeability test: measures lactulose‑mannitol ratio.
- Nutrient panels: iron, B12, vitamin D, zinc, and magnesium.
Management strategies focus on three pillars:
- Dietary reset: Gluten‑free, low‑FODMAP, or specific carbohydrate diets, depending on diagnosis.
- Microbiome restoration: Probiotic strains like Lactobacillus plantarum, prebiotic fibers, and occasional fecal microbiota transplantation for refractory IBD.
- Immune modulation: Vitamin D supplementation (2000‑4000 IU daily), omega‑3 fatty acids, and, when necessary, disease‑modifying drugs (e.g., mesalamine for ulcerative colitis).
Bottom Line and Next Steps
If you’re experiencing unexplained fatigue, joint pain, or recurring infections, consider that poor nutrient absorption might be nudging your immune system over the edge. A targeted work‑up can reveal hidden malabsorption, allowing you to act before an autoimmune condition fully manifests.
Start by tracking meals, noting any trigger foods, and requesting basic labs from your GP. From there, a gastroenterologist or functional medicine practitioner can guide you through the specific tests and interventions outlined above.
Frequently Asked Questions
Can leaky gut cause autoimmune disease on its own?
Leaky gut isn’t a diagnosis yet, but increased intestinal permeability can let antigens into the bloodstream, which may trigger or worsen autoimmunity in genetically‑susceptible people.
What’s the difference between celiac disease and gluten sensitivity?
Celiac disease is an autoimmune disorder with measurable antibodies and intestinal damage. Non‑celiac gluten sensitivity causes similar symptoms but lacks the immune markers and villous atrophy.
How long does it take for gut healing after starting a gluten‑free diet?
Most patients see symptom relief within weeks, but full villous regeneration can take 6‑24 months, especially if the diagnosis was delayed.
Are probiotics effective for reversing malabsorption?
Targeted probiotic strains can improve gut barrier function and reduce inflammation, which helps absorption. Effectiveness varies; a trial with Lactobacillus plantarum showed a 20% improvement in nutrient uptake in IBD patients.
Should I get tested for vitamin D even if I feel fine?
Yes. Vitamin D deficiency often presents silently but is a known risk factor for several autoimmune diseases. A simple blood test can guide supplementation before problems arise.

Terry Duke
Wow-what a thorough breakdown of how malabsorption can set off a cascade of immune reactions! Your explanation of the enzyme shortfalls, villi flattening, and microbial toxin interference really ties the pieces together, and I appreciate the clear bullet points that made it easy to follow. The link between leaky gut and systemic inflammation is especially eye‑opening; I hadn’t realized how zonulin levels could be a marker for both type 1 diabetes and multiple sclerosis. This definitely encourages me to look into my own diet and possibly get my vitamin D checked.